Applications of Borax Decahydrate
- Soap and Detergents
- Personal Care Products
- Metallurgical Fluxes
- Corrosion Inhibition
- Adhesives
- Wire Drawing
- Refractories
- Flame Retardant
- Buffer & Catalyst
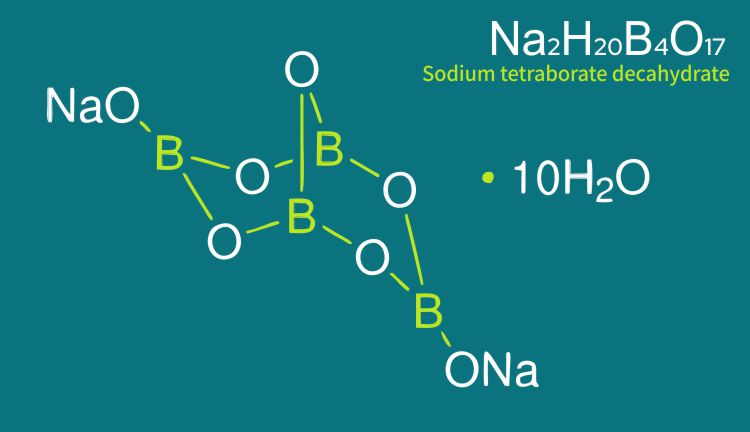
Borax decahydrate is the refined form of natural sodium borate. Composed of boric oxide (B2O3), sodium oxide, and water, it is a mild, alkaline salt, white and crystalline, with excellent buffering and fluxing properties. Available in powder or granular form, borax decahydrate is an important multi-functional source of B2O3, particularly for processes in which the simultaneous presence of sodium is beneficial.
Applications of Borax Decahydrate

Stability
Borax decahydrate is chemically stable under normal storage conditions. Borax decahydrate has a slight water vapor pressure which increases with warmer temperatures. This can cause crystallization at particle contact points, resulting in caking. Borax decahydrate will slowly lose water of crystallization if exposed to a warm, dry atmosphere. Conversely exposure to a humid atmosphere causes caking. When storing the product, care should therefore be taken to avoid wide fluctuations in temperature and humidity, and to ensure that the packaging is not damaged.
Buffering Action
Dissolved in water, borax decahydrate hydrolyzes to give a mildly alkaline solution. It is thus capable of neutralizing acids. It also combines with strong alkalis to form compounds of lower pH. The relatively constant pH of borax decahydrate solutions makes it an excellent buffering agent.